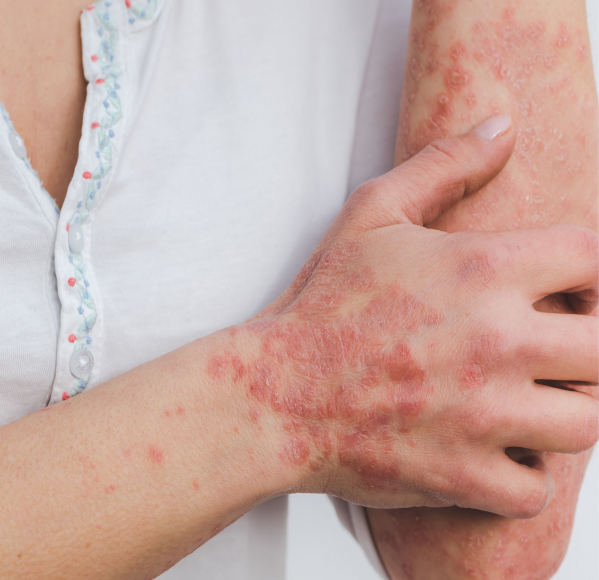
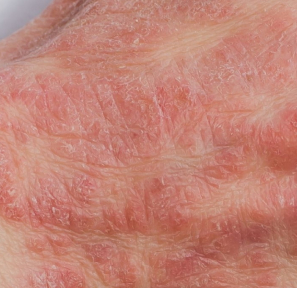
also known as atopic dermatitis, is a non-contagious skin condition that can affect people of all ages. Eczema can range from mild to severe, and is often characterized by red, itchy, dry, and cracked skin.
Areas of the body commonly affected include the face, inside of the elbows, or behind the knees, but eczema can appear anywhere on the body¹. During a “flare,” the symptoms can become painful with an increased urge to itch, resulting in changes in skin color and raw, bleeding blisters. The exact cause of eczema is unknown, but it can develop due to a combination of genes and individual triggers². Examples of triggers can include allergens, cosmetics, detergents, certain fabrics, weather changes (especially dry air), and even stress.
Doctors participating in the EASE clinical study are testing an investigational drug (EP262) to study its safety, tolerability, and effects in patients with eczema.
Eczema can develop by a combination of genetic and environmental factors, resulting in skin barrier dysfunction and dysregulation of the immune system. Mast cells (a type of immune cell present in the skin) have been implicated in the development of eczema skin lesions and severity³. MRGPRX2 is one of the receptors on mast cells, and activation of MRGPRX2 may play a role in eczema. EP262 is an experimental drug designed to block activators of the MRGPRX2 receptor, which may lead to fewer or less severe lesions.
EP262 represents a new, targeted approach to the treatment of eczema that is only available through participation in a clinical study.
If you have eczema you may be interested in learning more about the EASE study.